The Food and Drug Administration is putting out another warning about an at-home COVID-19 test that is not authorized for use in the U.S., but has a similar sounding name and package look as one that is OK to use in America.
Both have the name "Flowflex" and have identical name logos. But there are differences in product title and packaging to keep an eye out for.
The one to avoid -- which was recalled on January 9 -- is the "Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)." ACON said in its recall notice that it is an "unauthorized, adulterated and misbranded counterfeit product."
One way to identify it is that it comes in a dark blue box. Another is that that box is marked with the letters "CE" on the bottom of the front label. This "CE" mark means it is authorized for use the European Union and other countries, but not necessarily in the U.S.


The recalled one will also say on the side panel that it is from ACON Biotech (Hangzhou) Co., Ltd. in China.
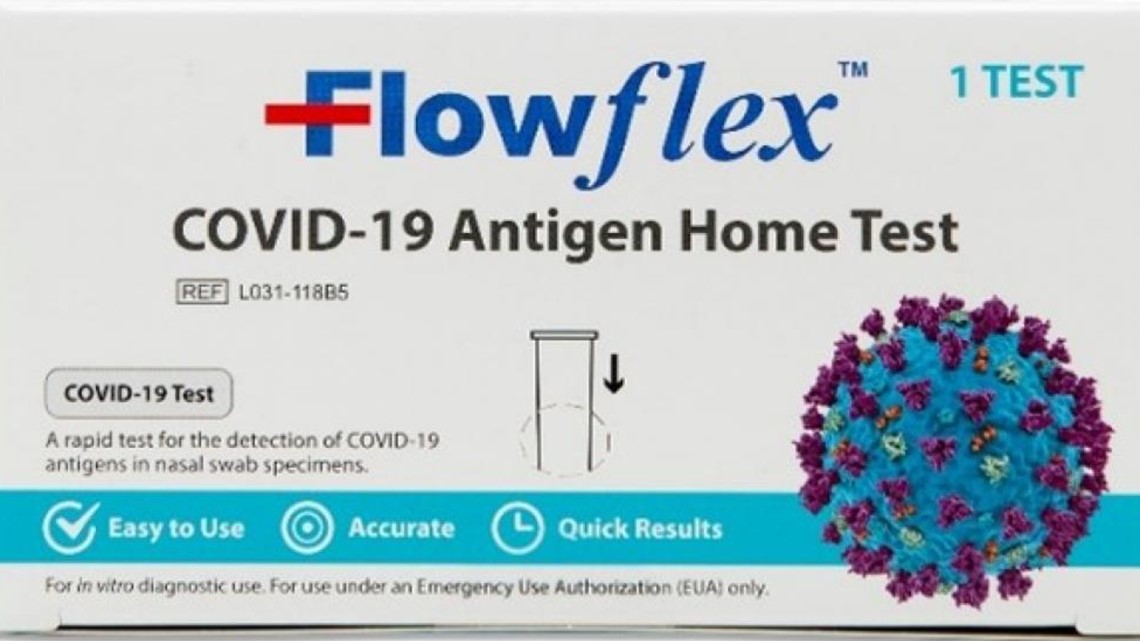
The one that has received the OK to use in the U.S. is the "Flowflex COVID-19 Antigen Home Test." This one comes in a white box and does not have the "CE" mark. And the side panel says it comes from ACON Laboratories, Inc. in San Diego.

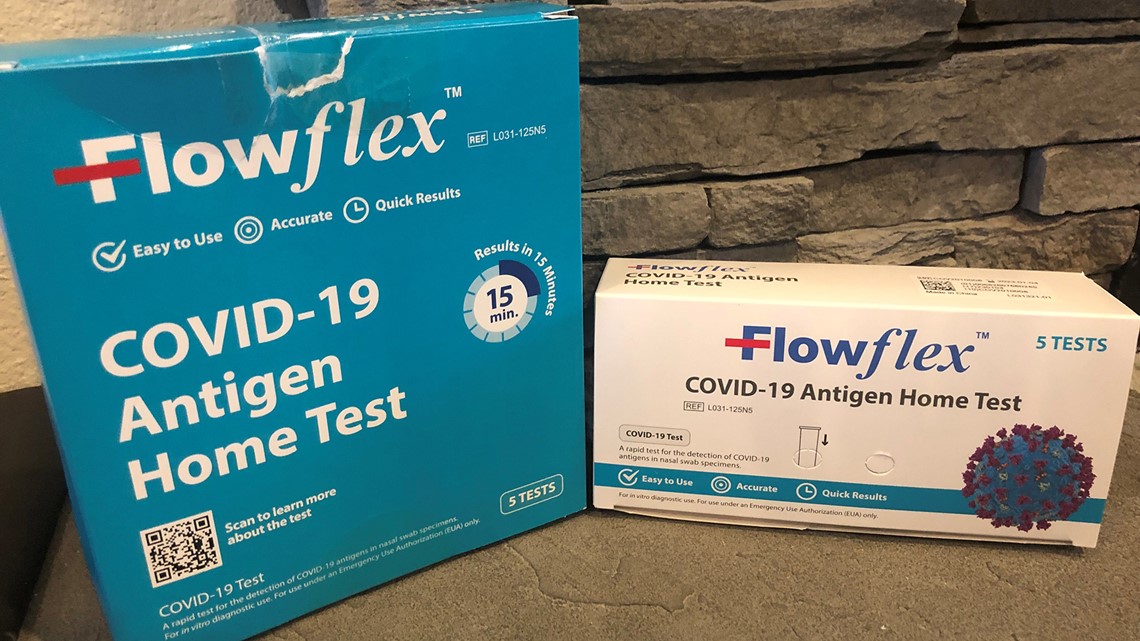
Some people have noted on social media that they believe they have bought the recalled one at Costco. They may be thrown by the bulk packaging. The one sold at Costco is the authorized “Flowflex COVID-19 Antigen Home Test" -- but it is sold as a five-pack and may have a white box placed in a larger turquoise box. That could be causing some confusion. The larger box also does not have the "CE" mark.

The recalled "Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)" may lead to inaccurate test results, according to the FDA. That includes false negative or false positive results.
A false negative would mean someone tests negative for COVID-19 when they are actually positive. That could lead the patient to not seek treatment for COVID-19.
A false positive would mean someone tests positive for COVID-19 when they are actually negative. That person could have some other ailment that they fail to get proper treatment for because they incorrectly believe they were infected with the coronavirus.
ACON Laboratories said it has not received reports of adverse events related to the recall.

